
リクルートの
転職スカウトサービス
リクルートの求職活動支援サービス共通で使える「レジュメ」に対応※。
登録すると、企業やエージェントから直接スカウトが届きます。
効率的に転職活動をしつつも、あなたの可能性を広げる企業との、新しい出会いを見つけましょう。
※利用できるサービスは説明ページをご確認ください。
アカウント&レジュメ移行のお願い
2ステップでカンタンに手続き完了!
①リクルートIDでログインもしくは新規登録
②これまでに登録したレジュメのデータを移行
これまでのサイトでは過去のスカウトやメッセージの確認・返信のみが可能です。
お知らせ
特徴

選ぶだけでレジュメが完成
あなたは経験や能力、希望条件を選んでいくだけ。
レジュメが完成すると、企業やエージェントからスカウトを受け取れます。

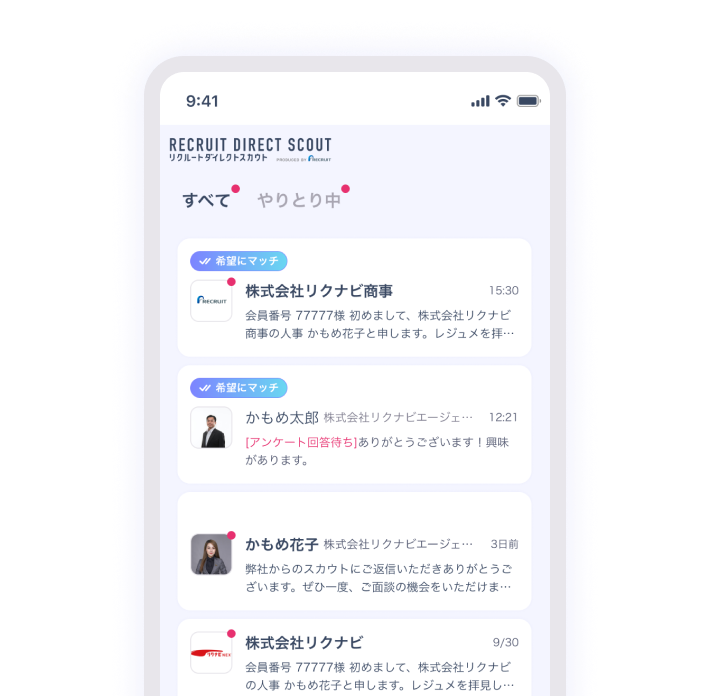

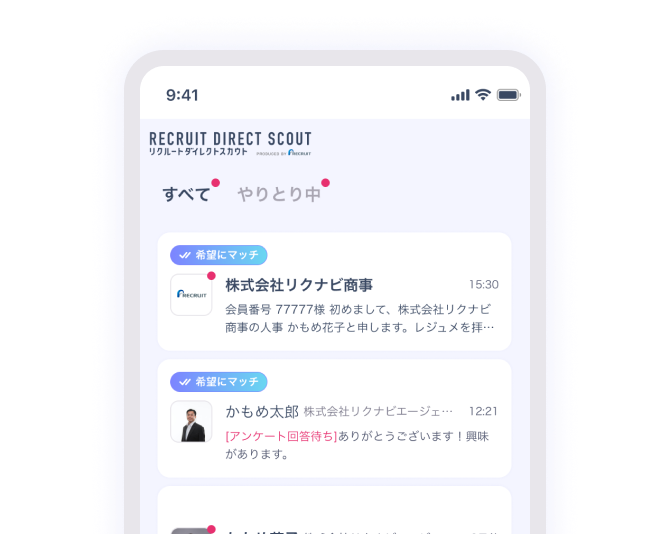
興味を持ったスカウトに返信
リクルートダイレクトスカウトがレジュメや求人情報を分析し、企業やエージェントにあなたをおすすめ。
あなたの興味にあったスカウトがきっと届きます。

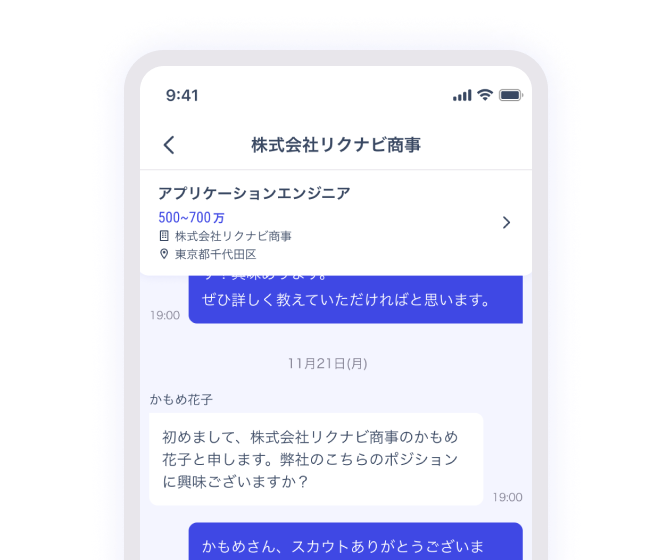
チャットでやりとり
企業やエージェントとのやりとりはチャットで。
気兼ねないやりとりを通じて、互いのことを深く理解できます。
よくあるご質問
企業やエージェントがスカウトを送りたい求職者を検索する際には、あなたが登録したレジュメの基本属性や持っているスキル、職務経験等が表示されます。これをもとに、企業やエージェントが、自身の持つ求人に合うかどうかを検討し、スカウトを送信します。
氏名、生年月日、住所など、あなたを特定できる情報は表示されません。
これらの個人情報は、あなたがスカウトに返信した際に初めて、企業やエージェントに公開されることになります。
ダイレクトスカウトは企業の担当者から直接スカウトを受け取ることができます。企業と直接コンタクトが取れるため効率的な転職活動ができます。
エージェントはあなたのスキルや経験を分析し、あなたの代わりに仕事を探してご提案します。自分では探せなかった、意外な企業との出会いがあるかもしれません。
スカウトに返信いただいたあとは、企業担当者もしくはエージェントとの面談を必ず受けることができます。
スカウトの内容や求人の詳細について確認したり、キャリアや転職に関する相談ができるよい機会です。ぜひご活用ください。
サイトリニューアルにあたり、ログイン方式およびレジュメが新しくなりました。
リニューアル前に登録いただいている方は、リニューアル後、登録済みの情報をリクルートIDや新しいレジュメに移行し、簡単に新サイトのご利用を開始いただけます。
